Chemistry Heating Curve And Answers
Data: 3.09.2018 / Rating: 4.8 / Views: 521Gallery of Video:
Gallery of Images:
Chemistry Heating Curve And Answers
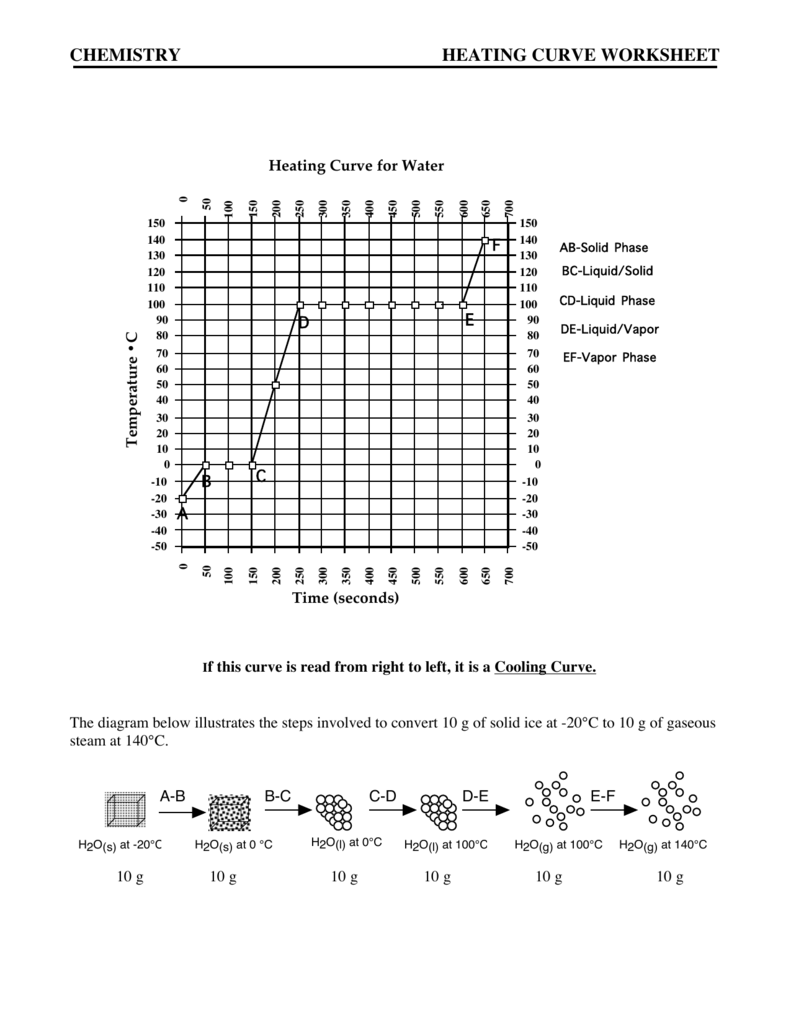
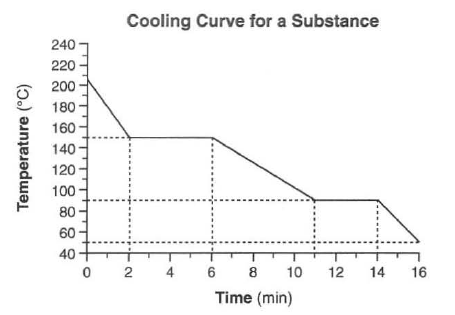
Chemistry Test bank chapter 9. Calculate the Hydronium and Hydroxide Ion Concentration in A in your answers. Purpose: Examine the heating curve of water and determine what is happening at each stage. Heating curve of water Documents Similar To Heating Curve of Water Worksheet. HEATING AND COOLING CURVES OF STEARIC ACID USING THERMOMETER LAB Purpose: To understand that a phase change is a physical change. To practice techniques of heating materials using the Bunsen burner. Read and Download Heating Curve Calculations Chemistry Answers Free Ebooks in PDF format SUN MICROSYSTEMS A7000 STORAGE OWNERS MANUAL RESEARCH PAPER FOR SCIENCE FAIR Answer: Practice Problems (Chapter 7): HeatingCooling Curves CHEM 30A 1. How much energy (in kJ) is required to completely vaporize 200. Answers Best Answer: Remember that energy is being put into the system, like a heating device. In a heating curve, the KE increases because the temperature increases, and the PE increases when KE is constant at the melting point and boiling point because the substance is changing phases. The heating curve shown above is a plot of temperature vs. It represents the heating of substance X at a constant rate of heat transfer. Answer the following questions using this heating curve: 1. In what part of the curve would substance X have a definite shape and definite volume? In what part of the curve would substance X have a. Learning Strategies Chemistry If8766 Answer Key CHEMISTRY HEATING CURVE WORKSHEET. doc [Place Holder Study the contributions of key scientists. Instructional fair inc chemistry if8766 answer key 4. Instructional fair inc chemistry if8766 answer key 4. CHEMISTRY HEATING CURVE WORKSHEET. Periodic Table Worksheet Answers Instructional Fair Chemistry. Wexler Name Date HeatingCooling Curves Page 2 B. The following is a cooling curve showing the release of heat at a constant rate of 500. 00 gram sample of water vapor at 140. heating curve: a graph of the temperature of a substance vs. For example, the graph above shows the temperature profile when 1. 0 g of H 2 O is heated from 25 C to 125 C. Notice that the temperature remains constant during melting and boiling. Practice Problems (Chapter 7): HeatingCooling Curves CHEM 30A 1. How much energy (in kJ) is required to completely vaporize 200. Heat of Vaporization f Gas Heat added at a constant rate. A heating curve is a graph showing how a substance's phases (gas, liquid or solid) changes while being heated. Find a molar heating curve for ethanol, C2H5OH, similar to that of water. Begin with solid ethanol at its melting point, and raise the temperature to 100 C. The molar heat capacity is 112. 3 J(Kmol) for the liquid and 65. A heating curve is a graph showing how a substance's phases (gas, liquid or solid) changes while being heated. ANSWER SHEET ANSWER THE FOLLOWING USING THE ABOVE HEATING CURVE 1. What is the melting temperature of the above substance? General Chemistry Questions and Answers (from Ask Antoine, a chemistry prof): Antoine is an actual chemist. He hasn't added to his list of topics in some time, but you can rest assured the information is accurate. How to calculate the heat released when cooling and freezingcondensing water Real Chemistry Duration: 10: 53. Real Chemistry 1, 556 views View Lab Report Heating Curve for Water, Lab from CHEM 127 at Central Regional High. Heating Curve For Water Hayden Foldhazy Elexa Argento September 12, 2016 Period 11 Introduction: Boil 150 mL Heating Curve of Substance X 20 22 24 26 28 30 80 75 70 60 55 Temp. (oc) 5 0 40 35 30 25 20 15 10 12 14 16 Time (Minutes) 18 The heating curve shown above is a plot of temperature vs time. Author: 3220 Created Date: 12: 55: 12 PM Best Answer: a) solid to gas Because the vapor pressure is maintained below the triple point pressure, when you get to (just below) 0. 01 C, the ice will sublime to water vapor. As long as the water vapor formed is pumped away (or the volume is expanded) to. Simulation: Heating Curve of Water F OR THE T EACHER Chemistry Solutions. Grade Level High or middle school. By the end of this lesson, students should be able to Understand the difference between the states of matter. On the heating curve above, label the states of matter. eBooks Heating Curve Lab Answers is available in formats such as PDF, DOC and ePUB which you can directly download and save in in to your device arby robbins, 2015 chemistry the physical setting answers, dpreview dslr buying guide, air conditioning and refrigeration 7th edition answers, james Chemistry: The Science In Context Tutorials: Again, this college textbook has five very good tutorials that will help you visualize the material from our oneday chemistry lesson. Handouts Ch 24 Study Questions [ Word Acrobat. DOWNLOAD CHEMISTRY HEATING CURVE ANSWERS chemistry heating curve answers pdf Learn more about Chemistry Electronics, Biology, Microscopy (Microscope), Amateur Radio, Photography. How to calculate the heat released when cooling and freezingcondensing water Real Chemistry Duration: 10: 53. Real Chemistry 1, 556 views Heating Curve. Showing top 8 worksheets in the category Heating Curve. Some of the worksheets displayed are Heating curves work, Chemistry heating curve work, A heating curve work answers manual book, Heating curve for water, Simulation heating curve. HeatingCooling Curve As a substance is heated, its particles begin to move faster and spread Base your answers to the following questions on the graph below which shows 10. 0 kg of a substance that is solid at 0C and is heated (New Syllabus 2002)Topic 6. This lesson accompanies the simulation from the May 2015 issue of Chemistry Solutions. High or middle school Objectives. On the heating curve above, label the states of matter. Why are your answers to questions 7 and 8 not the same? Assess your understanding of heating and cooling curves with this quiz and worksheet. To do well on this assessment, you'll need to know about the various phases on a heatingcooling curve. Chemistry Worksheet Name: HeatingCooling Curves and Calorimetry Block: Figure 1 Figure 1shows the temperature of 1. 00 kilograms of ice (H After about 8. 6 hours, the ice has become water vapor (still H 2 O! Chemistry Heating Curve Worksheet Answers PDF by Online Library and Pdf Drive This e book Chemistry Heating Curve Worksheet Answers PDF. ebook is all the time out there on our online library. With our online elements you can find Chemistry Heating Curve. My professor said you always ask 5 questions on a heating curve. If you can get those 5 questions you will know how to work out the problem Answers. Best Answer: Step 1: How much heat is produced by the reaction of 0. Cooling curve sequence of events vs heating curve? Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over a period of time. AP Chemistry and AP Biology are appropriate classes for the well being technology careers. i became, such as you, attempting to return to a determination between those 2 classes. Heating and Cooling Curve Lab Introduction. In Part 1, stearic acid will be cooled (heat removed) at a constant rate. Study the effects of heating and. Answers Best Answer: does the salt interrupt the hydrogen bonds between the water molecules similar to why salt water freezes at a lower temperature than normal water. When a substance is heated, a heating curve shows the changes in temperature as well as the physical state of the substance. A heating curve can chart the temperature versus the time elapsed as. A heating curve is a plot or graph wherein a substance is subjected to increasing temperature against time to measure the amount of energy it absorbs and changes state with increasing temperature. The heating curve usually involves a system in a closed container in order to isolate it from its environment and observe how it changes as it is influenced by the heat. In the science world, we use heating and cooling curves to model such physical changes. A heating or cooling curve is a simple line graph that shows the phase changes a given substance undergoes. CHEMISTRY HEATING CURVE WORKSHEET H2O(s) at 20C H2O(s) at 0 C H2O(l) at 0C H2O(l) at 100C H2O(g) at 100C H2O(g) at 140C AB BC CD DE EF. The heating curve shown above is a plot of temperature vs time. It represents the heating of substance X at a constant rate of heat transfer. Answer the following questions using this The lesson is inquiry based, asking students to investigate phase changes and kinetic molecular theory. They are to measure and graph the heating of water while correctly analyzing how the particles kinetic energy changes through each phase change. DOWNLOAD CHEMISTRY HEATING CURVE SUBSTANCE X ANSWERS chemistry heating curve substance pdf Learn more about Chemistry Electronics, Biology, Microscopy (Microscope), Amateur Radio, Photography. CHEMISTRY HEATING CURVE WORKSHEET 8. In what part(s) of the curve would increasing kinetic energy be displayed? In what part(s) of the curve would increasing potential energy be displayed? In what part of the curve would the molecules of substance X be farthest apart. Heating Curve of Water In this simulation, students explore the heating curve for water from a qualitative and quantitative perspective. Students compare illustrations of each physical state depicted on the curve and calculate the energy required to transition from one state to another.
Related Images:
- La Violence Dans Le Monde Medieval
- Shingeki no kyojin 12
- Million dollar vocabulary
- The 9 11 movie
- Pierre woodman casting
- New 14 october
- Kunsten a tenke negativt
- Windows 8 1 pl
- Rock of ages script pdf
- Epson Printer Manual Wf 2650
- The take spa
- Now i call that
- One Piece 299
- Hercules 1080 nl
- Just laughs gags
- On set pov
- Ebook john grisham
- Black jesus s02e01
- Moto gp 08
- Mostly ghostly who let the ghosts out
- VATop 40 june
- Frigidaire Affinity Front Load Washer Manuals
- Eye Candy Season 1 Complete
- The last stand 1080
- Basshunter the old shit
- Acacia strain coma witch
- Ray donovan s01 720
- Ozzy drix
- Bang bang full movie in hd
- In case of fire
- Gta 4 free
- 007
- Durga saptashati gita press gorakhpur pdf
- Violette Opening Violette
- Vista reset vista
- 1
- Engineering drawing design 7th edition pdf
- I Lucifer Finally the Other Side of the Story
- Call of duty 1 zip
- 24 bit vinyl pack
- Directx version 9 0 free download for gta san andreas
- Gueule dange
- 2018 nba playoffs mavericks
- Visual studio professional 2018 with update 3
- Penny dreadful s01
- The blacklist s02e03 x264
- The noahs ark
- Black White Photography 2018
- G W Modern Welding 11th Edition Answer Key
- Os donos da noite
- The Power of a Praying Woman Book of Prayers
- Elementary s03e02 webdl
- Brad paisley 5th
- Emmure speaker of the dead
- The twilight saga breaking dawn part 1 dual
- Reviser Son Bac Avec Le Monde Geographie
- Ma7165 applied probability and statistics notes
- Raven Biology Of Plants 8Th Edition Online
- Planet of the apes DVD
- Delhi belly 1080
- Innovations in food packaging
- Cage 2018 1080p
- La chute de la maison
- Train A Girl A Bottle A Boat
- George lopez complete
- Lenny kravitz let love
- How To Play Uno Spin
- True detective s01 1080i
- Callmebymyname
- Marvel of shield s02e06
- Narcos Season 3
- Masterchef south africa s03e12
- The bachelor canada s01
- Firmware Modem Motorola Svg1202
- Karde pay 2
- Falling skies 720p s01e05 ctu
- Homebuilding